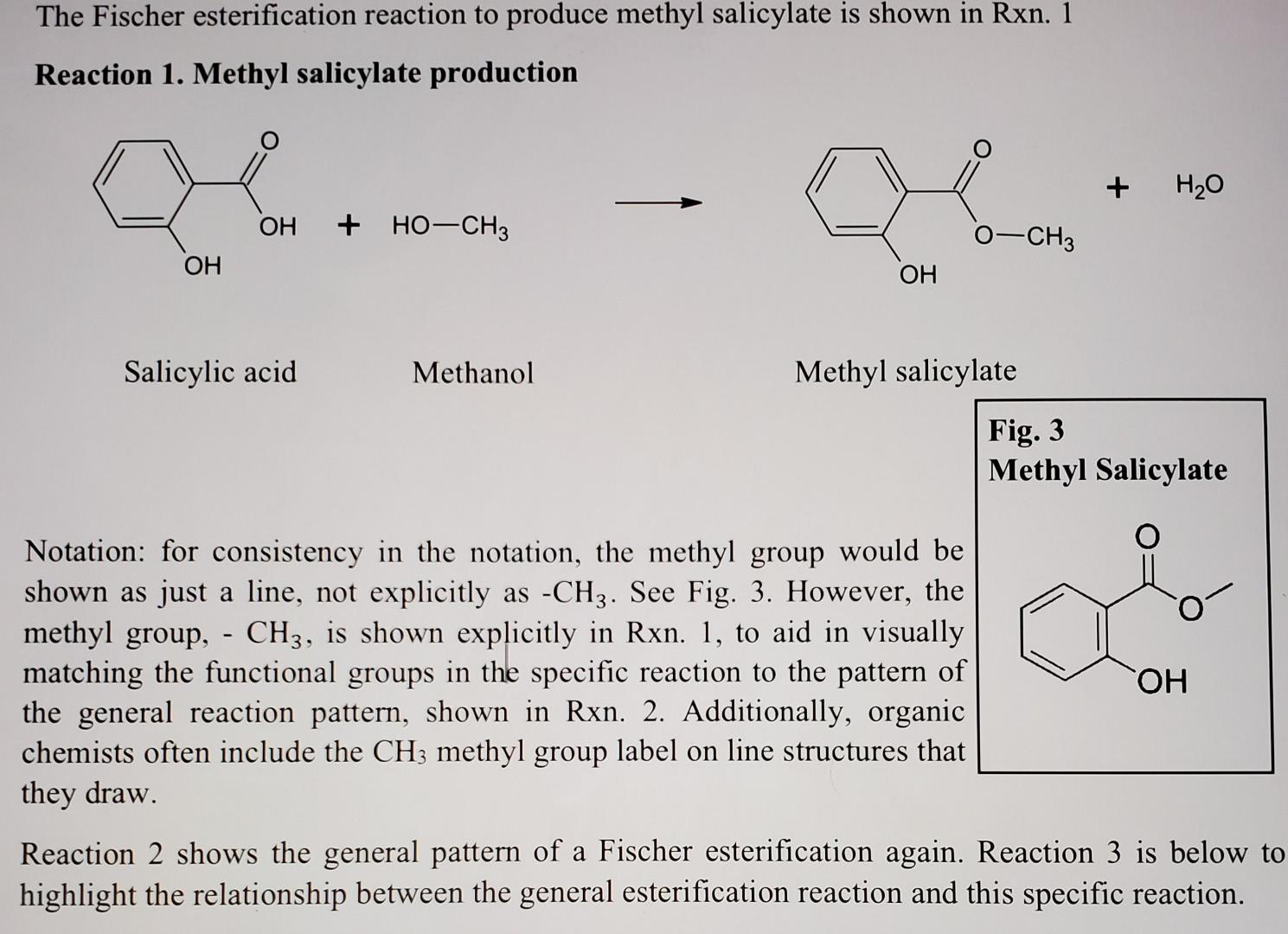
What Is The Theoretical Yield Of Aspirin
It’s easy to feel scattered when you’re juggling multiple tasks and goals. Using a chart can bring a sense of structure and make your daily or weekly routine more manageable, helping you focus on what matters most.
Stay Organized with What Is The Theoretical Yield Of Aspirin
A Free Chart Template is a useful tool for planning your schedule, tracking progress, or setting reminders. You can print it out and hang it somewhere visible, keeping you motivated and on top of your commitments every day.
What Is The Theoretical Yield Of Aspirin
These templates come in a variety of designs, from colorful and playful to sleek and minimalist. No matter your personal style, you’ll find a template that matches your vibe and helps you stay productive and organized.
Grab your Free Chart Template today and start creating a more streamlined, more balanced routine. A little bit of structure can make a big difference in helping you achieve your goals with less stress.
Solved DATA ANALYSIS What Is The Theoretical Yield Of Chegg
You calculate the theoretical yield of aspirin and then you use your actual yield to calculate the percent yield Here s how to calculate the theoretical yield of aspirin And here s how you calculate percent yield EXAMPLE If the theoretical yield of aspirin is 2 748 g and you obtained 2 47 g of aspirin what is your percent yield Solution Percent yield actual yield theoretical Purpose In this experiment you will synthesize aspirin from acetic anhydride and salicylic acid using phosphoric acid as a catalyst to speed up the reaction (it is not a reactant or product in the overall equation). The reaction for the synthesis is given below. C4H6O3 Acetic Anhydride + C7H6O3 Salicylic Acid H3PO4 → C9H8O4 Aspirin

Exercise 7 The Yield Of Aspirin Name Date Read Exercise 7 In Your
What Is The Theoretical Yield Of AspirinIn this case, both coefficients are 1 so we can just compare moles of each reactant. Next, use mols SA to find mols of aspirin formed (theoretical yield) Theoretical yield = 0.0154 mols SA x 1 mol aspirin / mol SA x 180.2 g aspirin/mol = 2.78 g aspirin. % yield = actual yield / theoretical yield (x100%) = 2.11 g / 2.78 g (x100%) = 75.9% yield. Solution Step 1 Find the molar mass of aspirin and salicylic acid From the periodic table Molar Mass of C 12 grams Molar Mass of H 1 grams Molar Mass of O 16 grams MM aspirin 9 x 12 grams 8 x 1 grams 4 x 16 grams MM aspirin 108 grams 8 grams 64 grams MM aspirin 180 grams
Gallery for What Is The Theoretical Yield Of Aspirin

SOLVED Calculate The Theoretical Yield Of Aspirin FW 180 158 G mol

Theoretical Yield Calculator Chemical Reaction Calculator
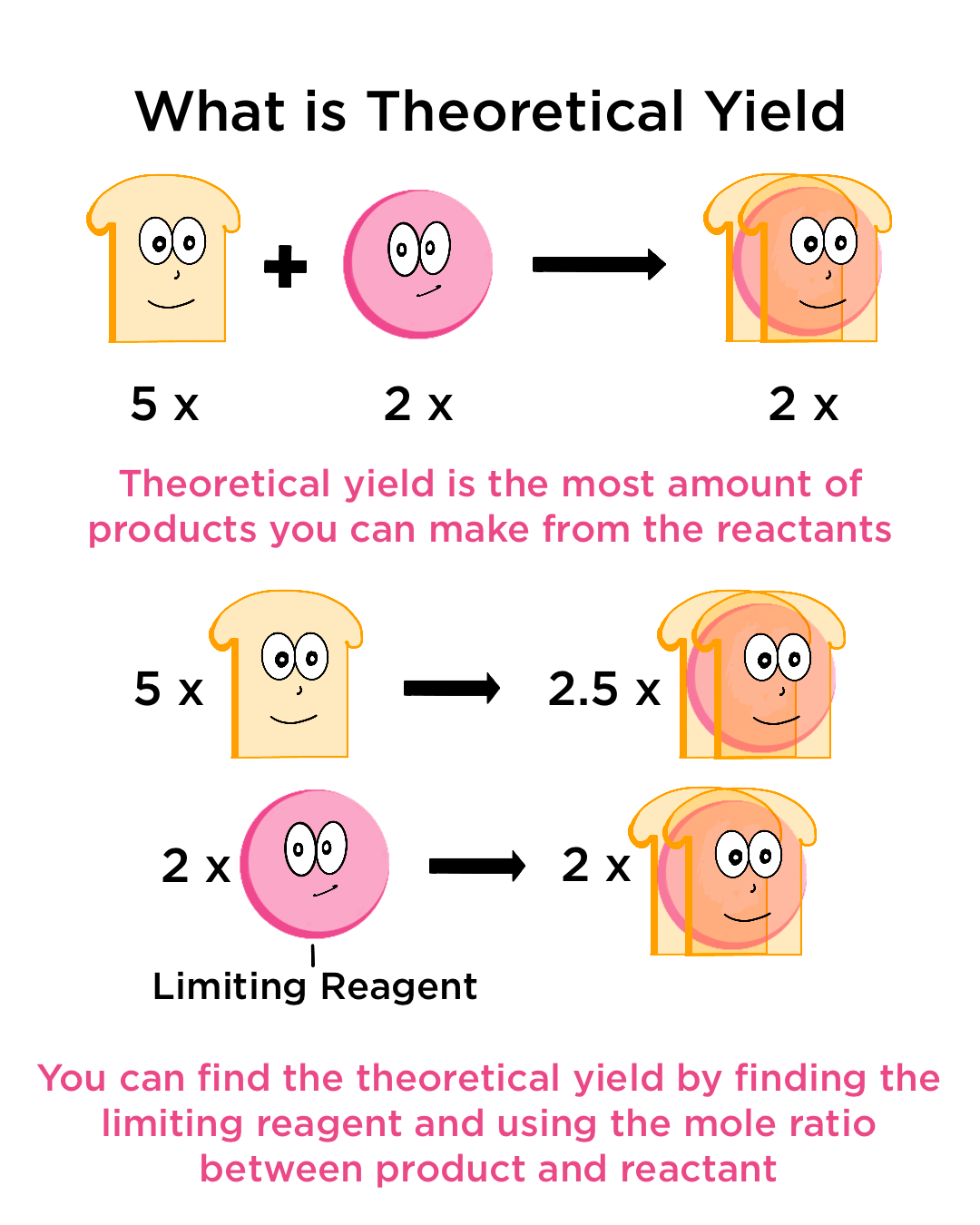
Theoretical Yield Definition Calculation Expii
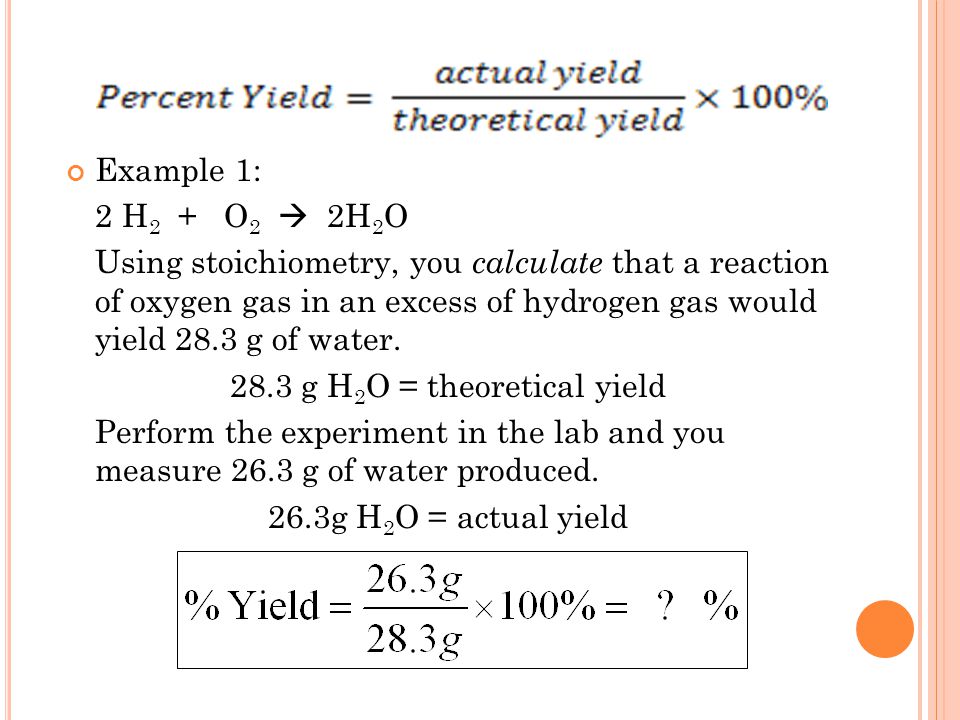
How To Calculate Yield Haiper
Solved Calculate The Theoretical Yield Of Aspirin If 3 687 G Of

How To Calculate The Percent Yield Formula Modeladvisor

How To Calculate The Theoretical Yield Of Aspirin How To Calculate
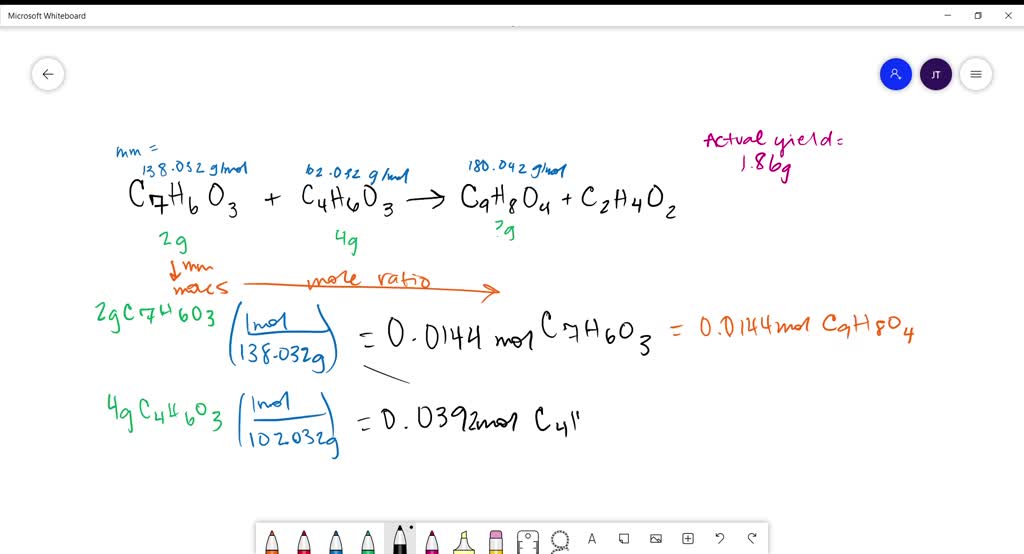
Aspirin Can Be Prepared From Salicylic Acid C H O SolvedLib
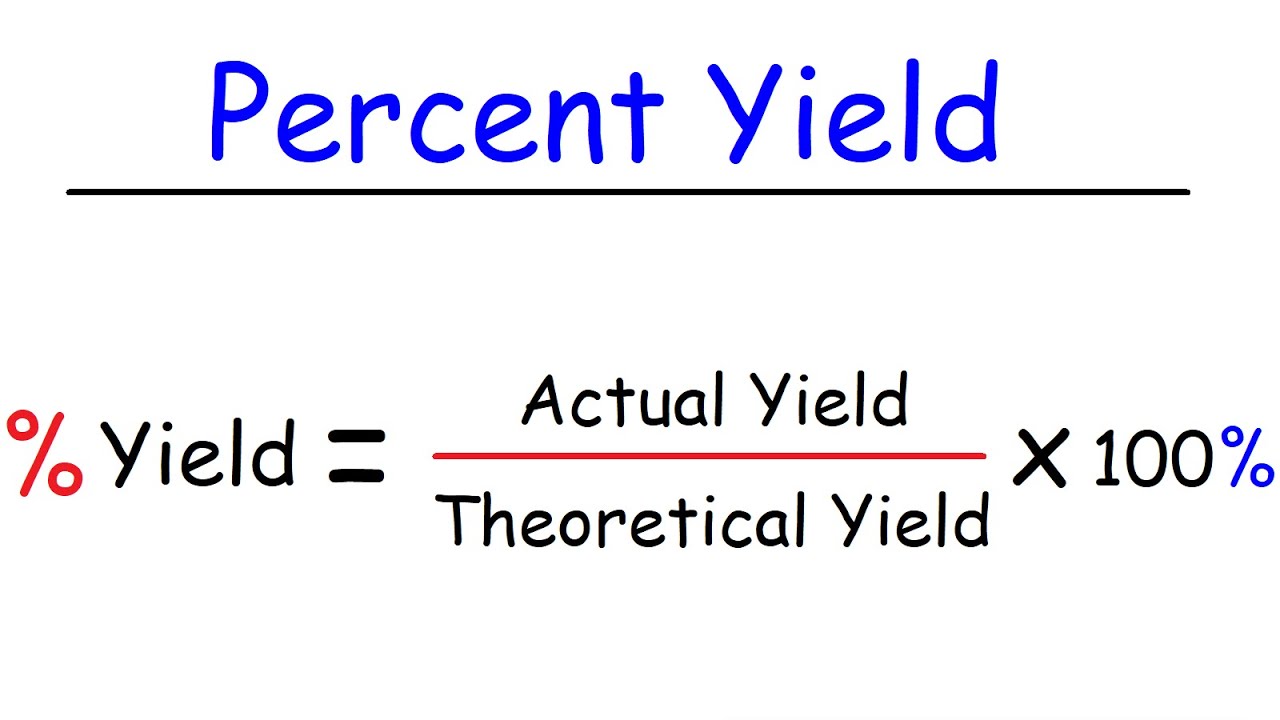
How To Calculate Theoretical Yield And Percent Yield YouTube
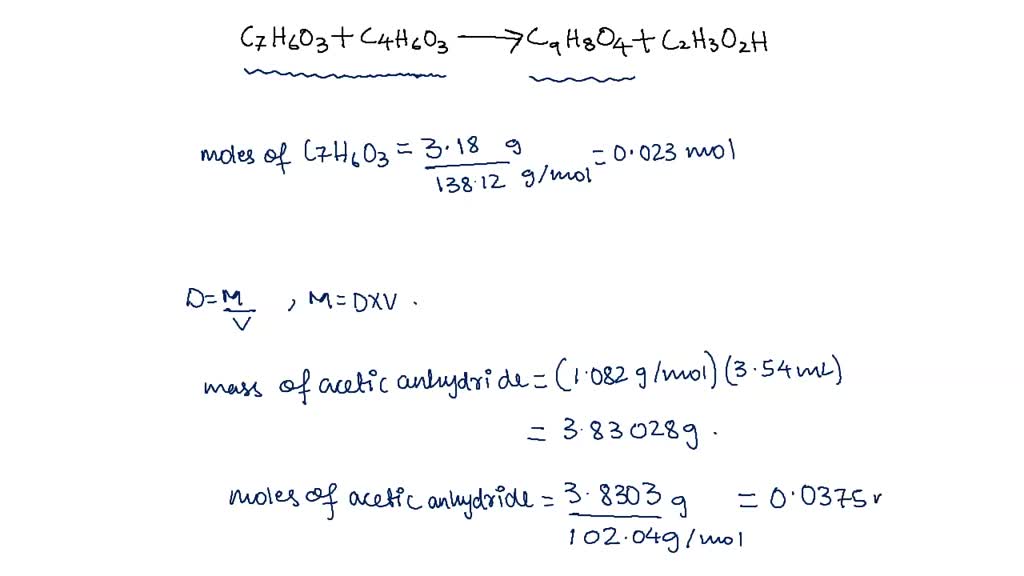
SOLVED Fe CEUS Raction Data And Results Preparation Of Aspirin Mass