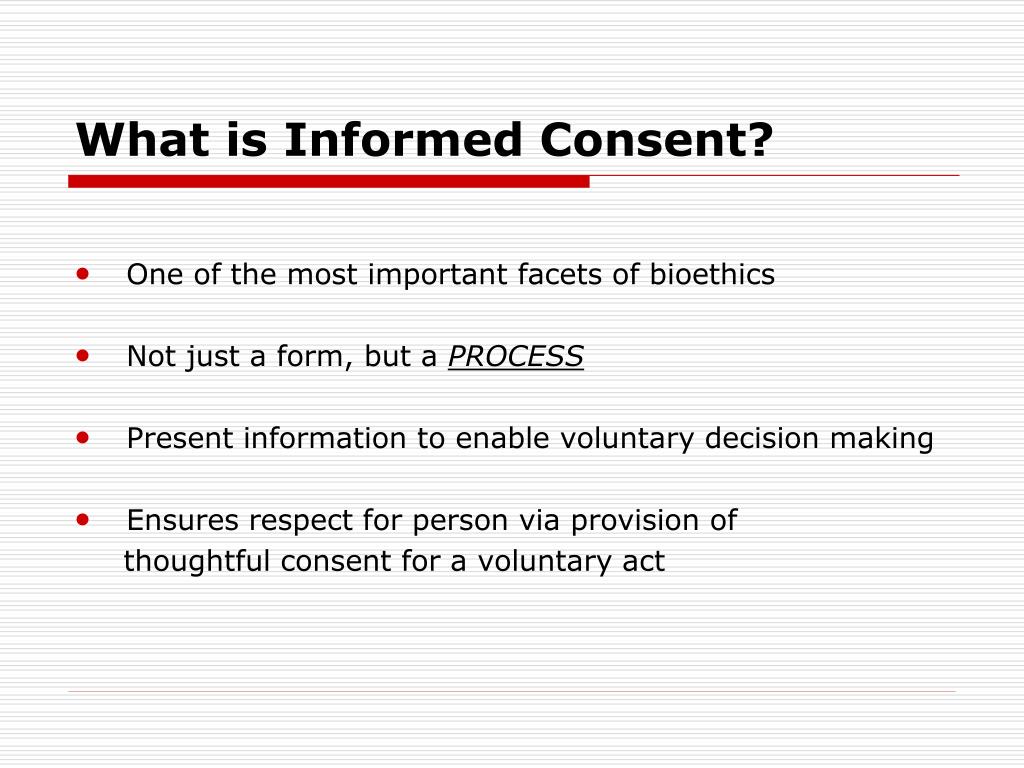
What Is Informed Consent In Research
It’s easy to feel overwhelmed when you’re juggling multiple tasks and goals. Using a chart can bring a sense of order and make your daily or weekly routine more manageable, helping you focus on what matters most.
Stay Organized with What Is Informed Consent In Research
A Free Chart Template is a great tool for planning your schedule, tracking progress, or setting reminders. You can print it out and hang it somewhere visible, keeping you motivated and on top of your commitments every day.
What Is Informed Consent In Research
These templates come in a variety of designs, from colorful and playful to sleek and minimalist. No matter your personal style, you’ll find a template that matches your vibe and helps you stay productive and organized.
Grab your Free Chart Template today and start creating a smoother, more balanced routine. A little bit of structure can make a big difference in helping you achieve your goals with less stress.

Informed Consent In Research YouTube
Web Mar 22 2018 nbsp 0183 32 When conducting clinical research the obtaining of informed consent is required Informed consent is a procedure through which a competent subject after having received and understood all the research related information can voluntarily provide his or her willingness to participate in a clinical trial INTRODUCTION. Informed consent is one of the primary principles on which the framework of protections for human subjects in research is built. In the United States, informed consent was codified in law via the statutes at 45 CFR 46 and 21 CFR 50 of the Code of Federal Regulations, yet the intellectual scaffolding on which it has been built …

What Is Informed Consent In Research Document Samples
What Is Informed Consent In Research;Informed consent is fundamental to the ethical and legal doctrines respecting research participants’ voluntary participation in clinical research, enshrined in such documents as the 1947 Nuremberg Code; reaffirmed in the 1964 Declaration of Helsinki, revised in 1975, and the 1978 Belmont Report; and... Web The informed consent process involves three key features 1 disclosing to potential research subjects information needed to make an informed decision 2 facilitating the understanding of what has been disclosed and 3 promoting the voluntariness of the decision about whether or not to participate in the research
Gallery for What Is Informed Consent In Research

What Is Informed Consent And Why It Is Important To Research Publication
2018 06 Sample Informed Consent Quantitative pdf Informed Consent Research Methods

What Is Informed Consent In Research Document Samples

5 Common Challenges When Obtaining Informed Consent In Clinical Research

PDF Informed Consent In Research

Informed Consent In Research Prof Oamr Kasule

Ssurvivor Example Of Informed Consent Form For Research
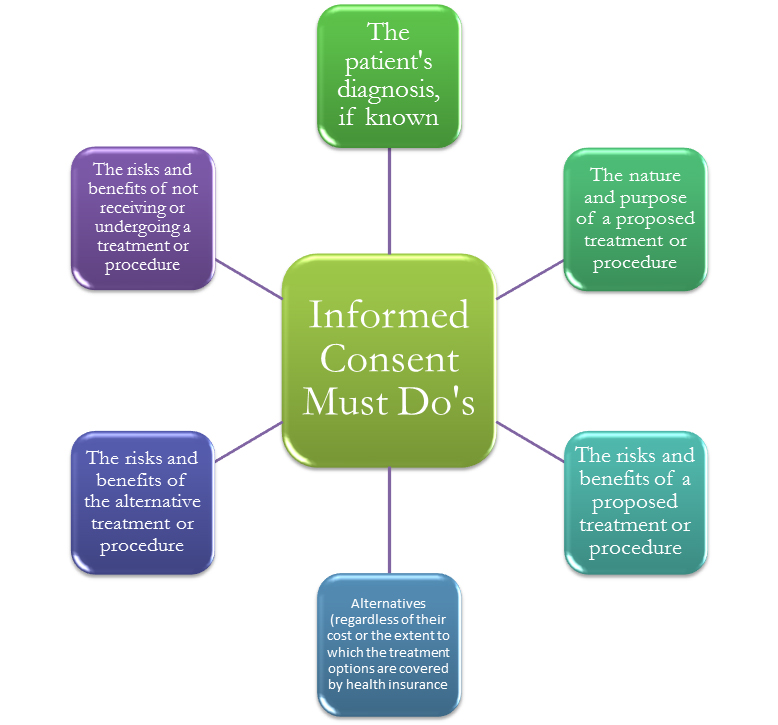
Informed Consent In Clinical Trials 5 New Trends And Aspects
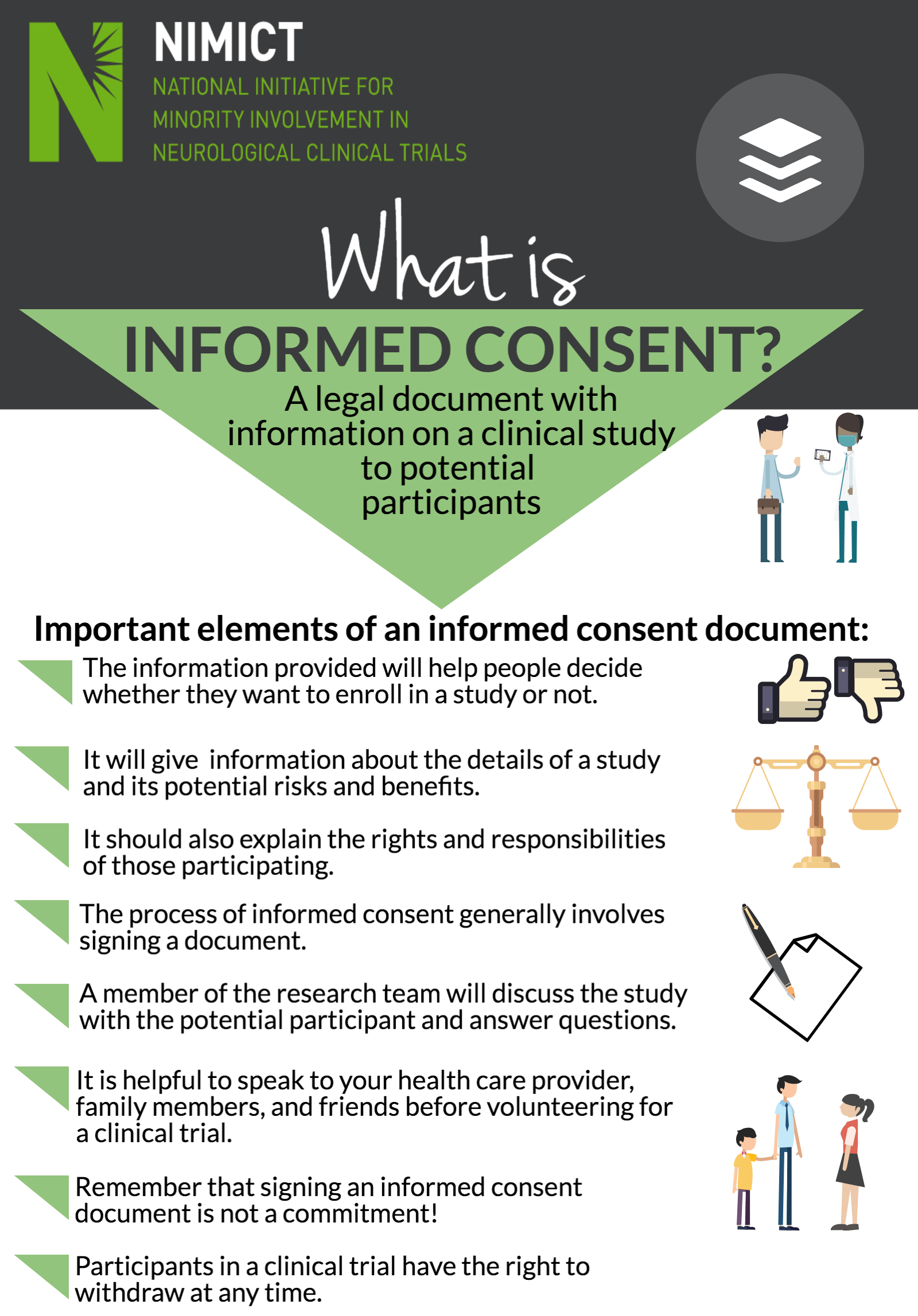
Informed Consent NIMICT

ND Example Consent PSY 30160 Experimental Psychology II Methods StuDocu
