Stoichiometry Practice Problems Worksheet
It’s easy to feel scattered when you’re juggling multiple tasks and goals. Using a chart can bring a sense of order and make your daily or weekly routine more manageable, helping you focus on what matters most.
Stay Organized with Stoichiometry Practice Problems Worksheet
A Free Chart Template is a useful tool for planning your schedule, tracking progress, or setting reminders. You can print it out and hang it somewhere visible, keeping you motivated and on top of your commitments every day.
Stoichiometry Practice Problems Worksheet
These templates come in a range of designs, from colorful and playful to sleek and minimalist. No matter your personal style, you’ll find a template that matches your vibe and helps you stay productive and organized.
Grab your Free Chart Template today and start creating a more streamlined, more balanced routine. A little bit of structure can make a huge difference in helping you achieve your goals with less stress.
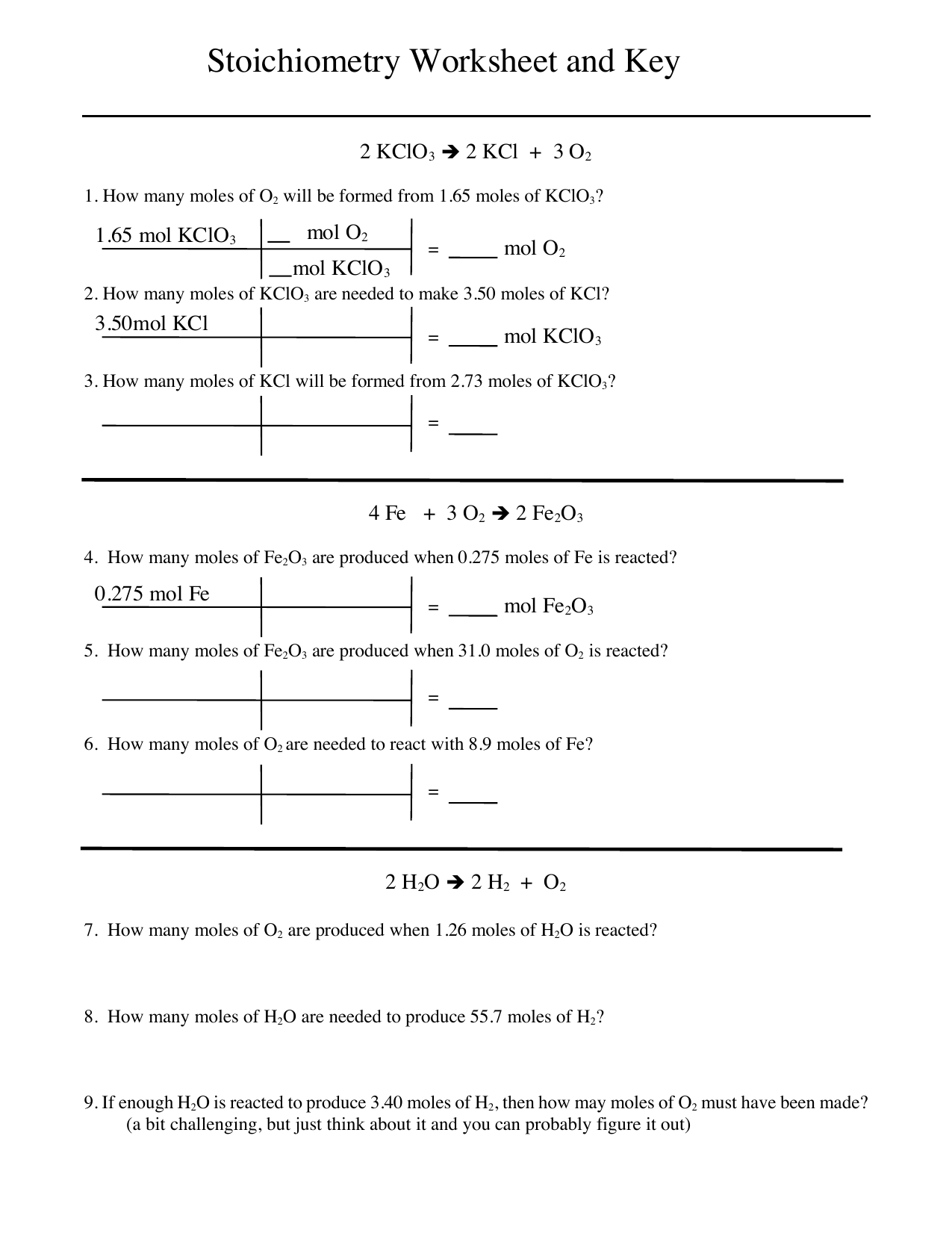
Stoichiometry Worksheet Answers Mole Unit Iron
PROBLEM 5 2 1 1 5 2 1 1 Write the balanced equation and determine the information requested Don t worry about state symbols in these reactions The number of moles and the mass in grams of chlorine Cl 2 required to react with 10 0 g of sodium metal Na to produce sodium chloride NaCl The number of moles and the mass in milligrams of Q4. Given the following reaction: H2SO4 + Na2CO3 → Na2SO4 +H2O + CO2 H 2 S O 4 + N a 2 C O 3 → N a 2 S O 4 + H 2 O + C O 2. Calculate the molarity of the H2SO4 H 2 S O 4 solution if it takes 40.0 mL of H2SO4 H 2 S O 4 to neutralize 46.7 mL of a 0.364 M Na2CO3 N a 2 C O 3 solution.
Stoichiometry Problems Worksheet 1 Answers Worksheets
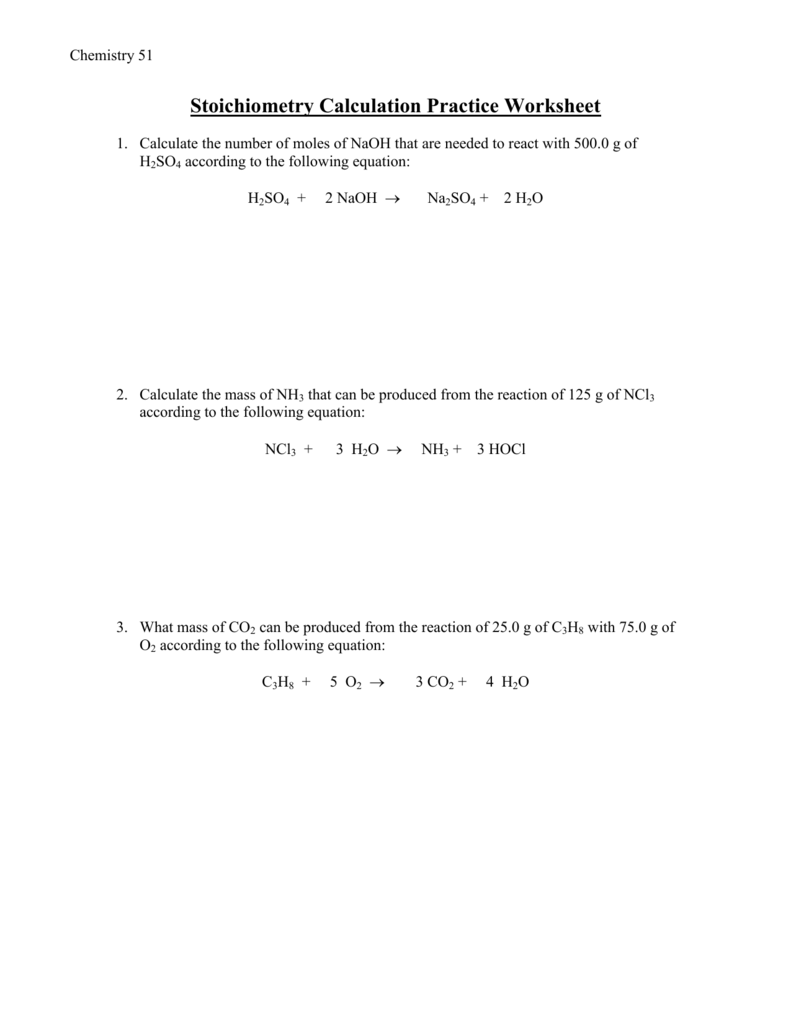
Stoichiometry Practice Problems Worksheet1. Calculate the number of moles of NaOH that are needed to react with 500.0 g of H2SO4 according to the following equation: H2SO4 + 2 NaOH Na2SO4 + 2 H2O ANS: 10.19 mol 2. Calculate the mass of NH3 that can be produced from the reaction of 125 g of NCl3 according to the following equation: NCl3 + 3 H2O This is a comprehensive end of chapter set of practice problems on stoichiometry that covers balancing chemical equations mole ratio calculations limiting reactants and percent yield concepts The links to the corresponding topics are given below The Mole and Molar Mass Molar Calculations Percent Composition and Empirical Formula
Gallery for Stoichiometry Practice Problems Worksheet

36 Chemfiesta Stoichiometry Practice Worksheet Answers Support worksheet

Stoichiometry Practice Problems Worksheet For 10th Higher Ed Lesson Planet

Stoichiometry Practice Worksheet

FREE 9 Sample Stoichiometry Worksheet Templates In MS Word PDF
Stoichiometry Practice 2 Worksheet Answers Koyumprogram

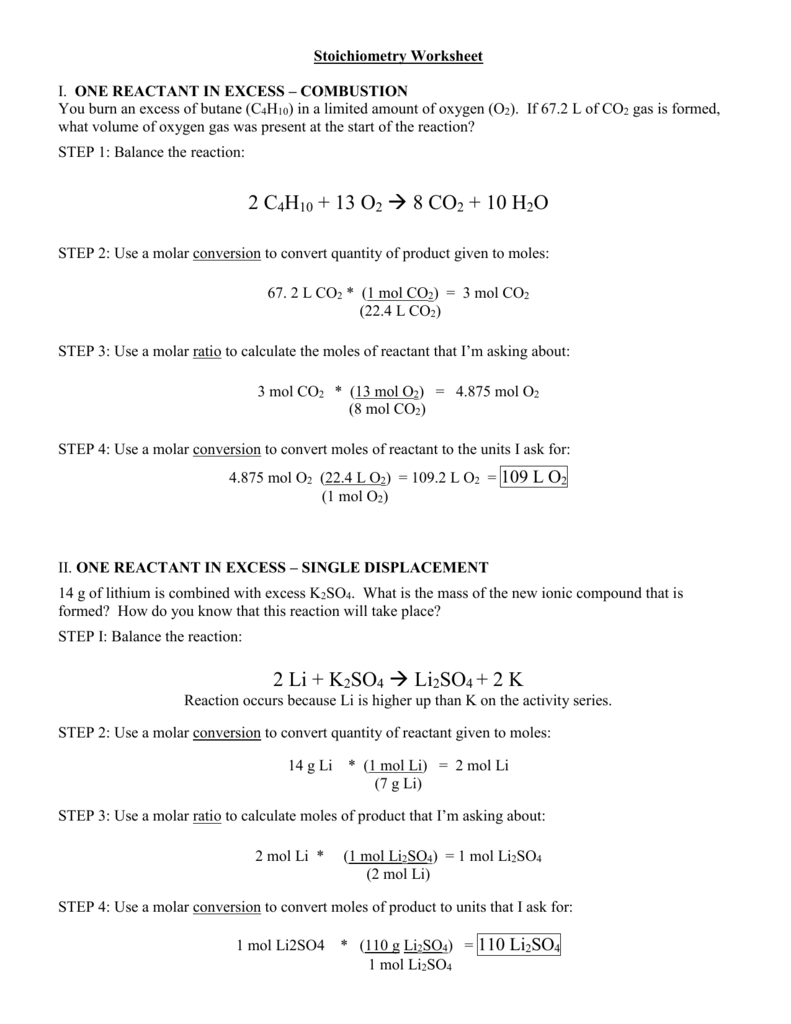
Stoichiometry Worksheet 2
Stoichiometry Worksheet With Answers

50 Stoichiometry Problems Worksheet Answers

Stoichiometry Practice Problems 1

Stoichiometry Worksheet 1
