How To Calculate Average Atomic Mass Of An Element
It’s easy to feel scattered when you’re juggling multiple tasks and goals. Using a chart can bring a sense of structure and make your daily or weekly routine more manageable, helping you focus on what matters most.
Stay Organized with How To Calculate Average Atomic Mass Of An Element
A Free Chart Template is a useful tool for planning your schedule, tracking progress, or setting reminders. You can print it out and hang it somewhere visible, keeping you motivated and on top of your commitments every day.

How To Calculate Average Atomic Mass Of An Element
These templates come in a variety of designs, from colorful and playful to sleek and minimalist. No matter your personal style, you’ll find a template that matches your vibe and helps you stay productive and organized.
Grab your Free Chart Template today and start creating a smoother, more balanced routine. A little bit of structure can make a huge difference in helping you achieve your goals with less stress.
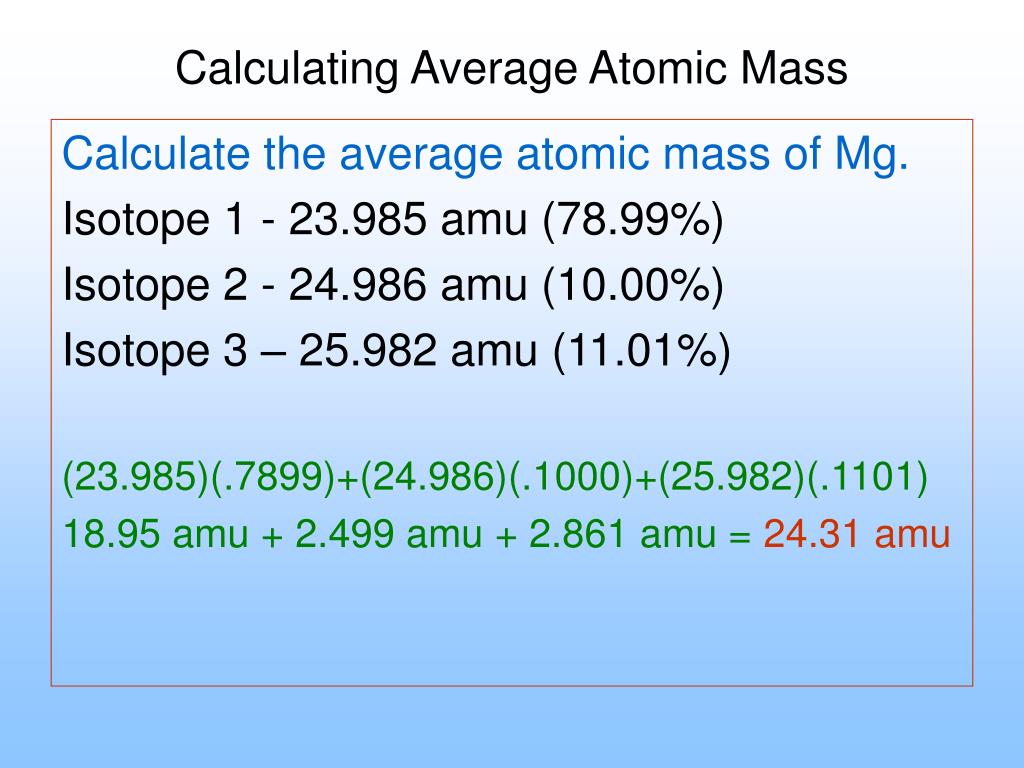
PPT Average Atomic Mass PowerPoint Presentation Free Download ID
The equation of average atomic mass is small text AM text f 1 text m 1 text f 2 text m 2 text f n text m n AM f1m1 f2m2 fnmn where AM text AM AM Average atomic mass f n rm f n f n Natural abundance of nth isotope and Average mass = (% large) x (mass large) + (% small) x (mass small) Average mass = 0.8 x 300 + 0.2 x 50 = 250 g. So, the average mass is closer to the mass of the larger tomato because it is more abundant. Now, we do the same thing for atoms. The mass of each isotope is multiplied by its percentage the products are summed up.
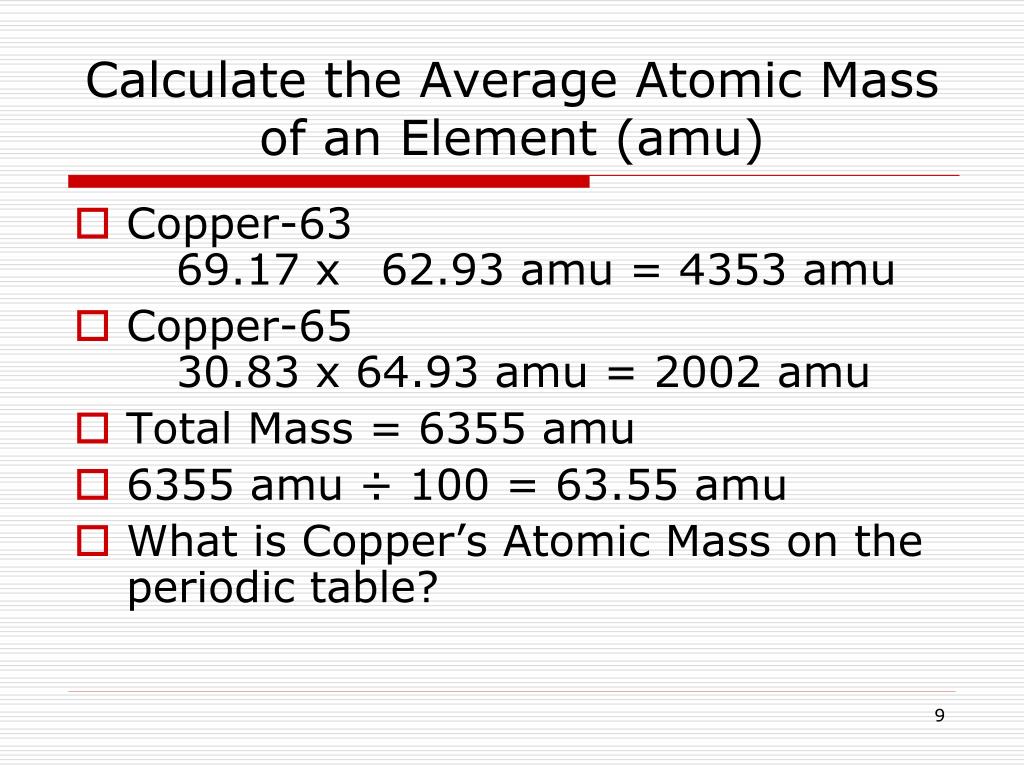
PPT Average Atomic Mass PowerPoint Presentation Free Download ID
How To Calculate Average Atomic Mass Of An ElementYou can calculate the atomic mass (or average mass) of an element provided you know the relative abundance (the fraction of an element that is a given isotope), the element's naturally occurring isotopes, and the masses of those different isotopes. We can calculate this by the following equation: Were you to simply calculate the arithmetic average of the precise atomic masses you would get 36 34 969 36 966 2 35 968amu 34 969 36 966 2 35 968 amu Clearly the actual average atomic mass from the last column of the table is significantly lower
Gallery for How To Calculate Average Atomic Mass Of An Element

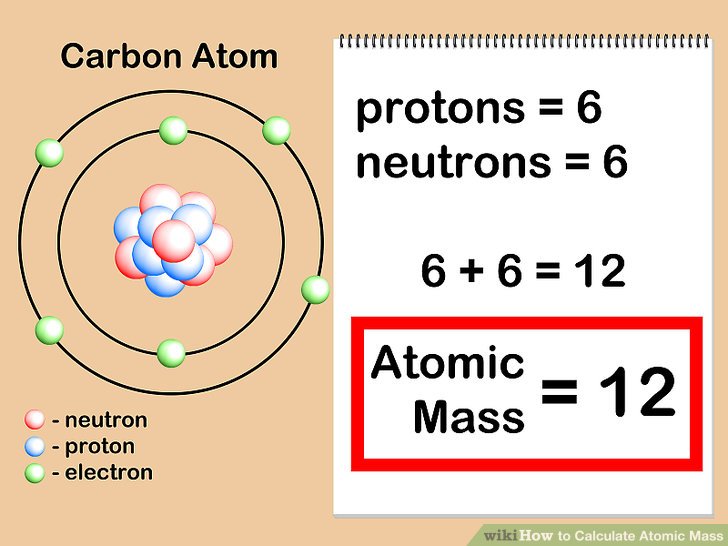
How To Find Average Atomic Mass 8 Steps with Pictures WikiHow
Periodic Table Element With Atomic Mass And Atomic Number

3 Clear And Easy Ways To Calculate Atomic Mass WikiHow
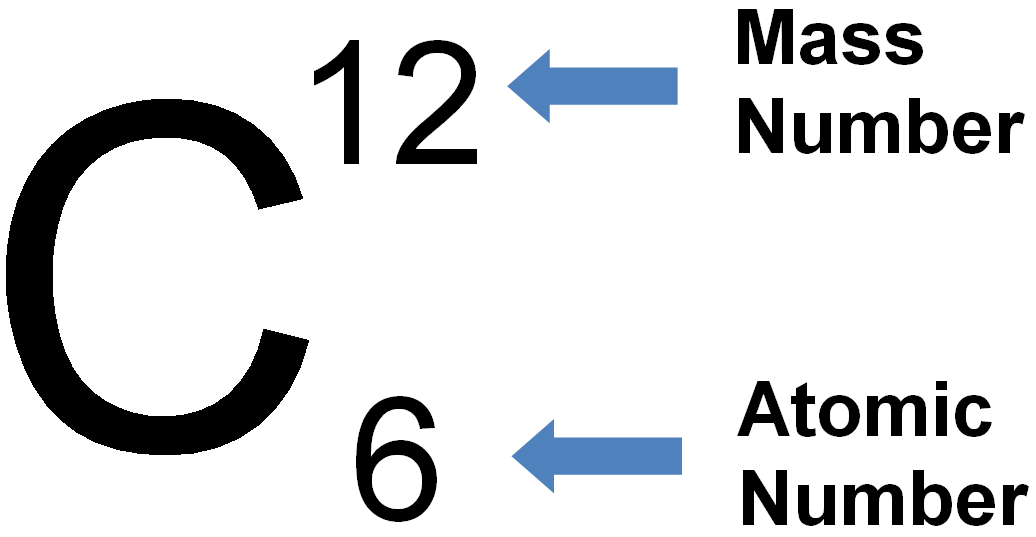
Periodic Table Showing Mass Number And Atomic Number Periodic Table

How To Calculate The Atomic Mass Of An Isotope Average Atomic Mass
Periodic Table Element With Atomic Mass And Atomic Number
Isotopes
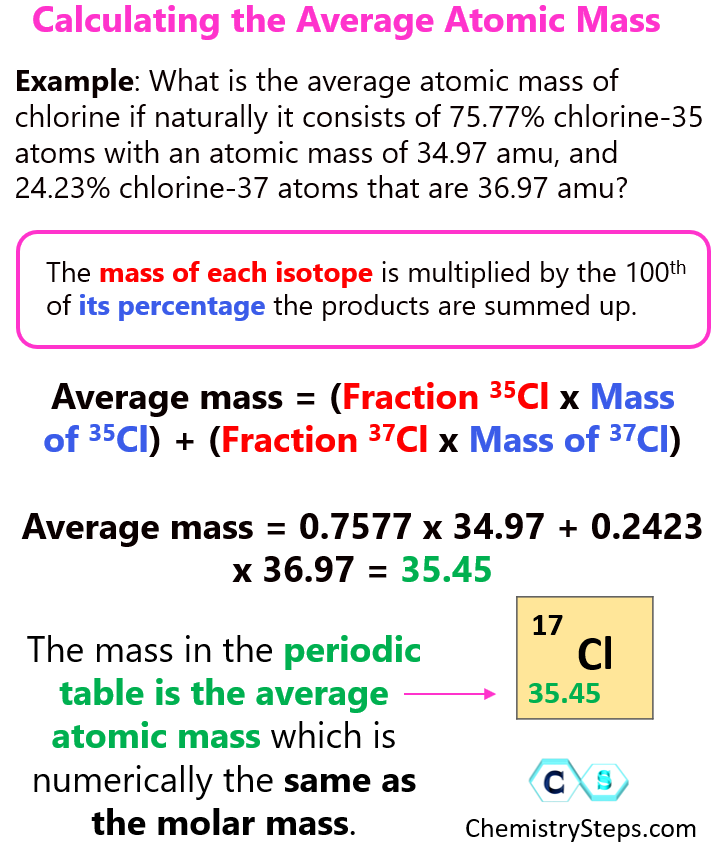
How To Calculate The Average Atomic Mass Chemistry Steps
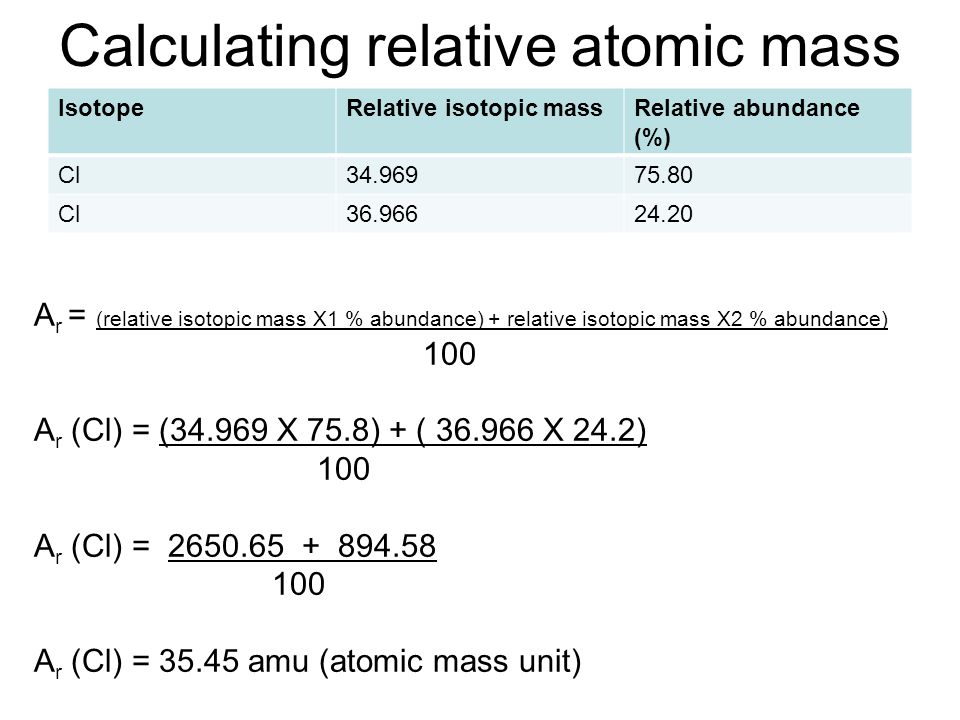
How To Find Atomic Mass And Number Of Elements

2 6 Calculating Average Atomic Mass YouTube
.PNG)